How To Find The Density Of Something
Density and Percent Compositions
- Page ID
- 350
Which ane weighs more, a kilogram of feathers or a kilogram of bricks? Though many people will say that a kilogram of bricks is heavier, they really counterbalance the aforementioned! However, many people are defenseless up by the concept of density, which causes them to answer the question incorrectly. A kilogram of feathers clearly takes up more space, but this is because it is less "dense." But what is density, and how can we determine information technology?
Introduction
Density (\(\rho\)) is a physical belongings found past dividing the mass of an object by its volume. Regardless of the sample size, density is always constant. For example, the density of a pure sample of tungsten is always 19.25 grams per cubic centimeter. This means that whether you have 1 gram or one kilogram of the sample, the density volition never vary. The equation is every bit follows:
\[ Density = \dfrac{Mass}{Volume} \]
or just
\[\rho = \dfrac{m}{v} \label{dens}\]
Based on Equation \(\ref{dens}\), information technology'due south articulate that density can, and does, vary from element to element and substance to substance due to differences in the relation of mass and volume. Permit united states interruption information technology down 1 step farther. What are mass and volume? Nosotros cannot understand density until nosotros know its parts: mass and book. The following two sections will teach y'all all the information you need to know about book and mass to properly solve and manipulate the density equation.
Mass
Mass concerns the quantity of matter in an object. The SI unit for mass is the kilogram (kg), although grams (g) are commonly used in the laboratory to mensurate smaller quantities. Often, people mistake weight for mass. Weight concerns the force exerted on an object as a function of mass and gravity. This can be written as
\[\text{Weight} = \text{mass} \times \text{gravity}\]
\(Weight = {m}{yard}\)
Hence, weight changes due to variations in gravity and acceleration. For example, the mass of a 1 kg cube will continue to be 1 kg whether information technology is on the top of a mountain, the bottom of the sea, or on the moon, simply its weight will differ. Another important difference between mass and weight is how they are measured. Weight is measured with a scale, while mass must be measured with a remainder. Just as people confuse mass and weight, they likewise misfile scales and balances. A balance counteracts the effects of gravity while a scale incorporates it. There are two types of balances found in the laboratory: electronic and manual. With a manual residual, you find the unknown mass of an object past adjusting or comparison known masses until equilibrium is reached.
Volume
Volume describes the quantity of three dimensional space than an object occupies. The SI unit for volume is meters cubed (miii), but milliliters (mL), centimeters cubed (cmiii), and liters (L) are more than mutual in the laboratory. At that place are many equations to find volume. Here are merely a few of the easy ones:
Volume = (length)3 or (length)(width)(height) or (base area)(pinnacle)
Density: A Further Investigation
We know all of density'south components, and then let's take a closer look at density itself. The unit most widely used to limited density is g/cm3 or 1000/mL, though the SI unit of measurement for density is technically kg/k3. Grams per centimeter cubed is equivalent to grams per milliliter (one thousand/cm3 = m/mL). To solve for density, simply follow the equation d = grand/v. For example, if you had a metal cube with mass 7.0 g and volume 5.0 cm3, the density would exist
\[\rho = \dfrac{7\,yard}{5\,cm^3}= 1.4\, g/cm^iii\]
Sometimes, you take to convert units to become the correct units for density, such as mg to g or inthree to cm3.
Density can be used to assist identify an unknown chemical element. Of form, yous have to know the density of an element with respect to other elements. Below is a table list the density of a few elements from the Periodic Tabular array at standard conditions for temperature and pressure, or STP corresponding to a temperature of 273 K (0° Celsius) and i atmosphere of pressure level.
| Chemical element Proper name and Symbol | Density (g/cmthree) | Atomic Number |
|---|---|---|
| Hydrogen (H) | 0.000089 \((8.nine \times 10^{-5})\) at 0 °C and i Atm. pressumre | 1 |
| Helium (He) | 0.000164 \((1.64 \times x^{-4})\) at 0 °C and 1 Atm. pressure | ii |
| Aluminum (Al) | 2.seven | 13 |
| Zinc (Zn) | 7.13 | 30 |
| Tin (Sn) | 7.31 | 50 |
| Iron (Iron) | vii.87 | 26 |
| Nickel (Ni) | 8.ix | 28 |
| Cobalt (Co) | 8.9 | 27 |
| Copper (Cu) | viii.96 | 29 |
| Silver (Ag) | 10.5 | 47 |
| Lead (Pb) | 11.35 | 82 |
| Mercury (Hg) | 11.55 | 80 |
| Gold (Au) | nineteen.32 | 79 |
| Platinum (Pt) | 21.45 | 78 |
| Osmium (Os) | 22.6 | 76 |
As tin can be seen from the table, the almost dense element is Osmium (Bone) with a density of 22.six g/cmiii. The least dense chemical element is Hydrogen (H) with a density of 0.09 m/cm3.
Density and Temperature
Density generally decreases with increasing temperature and likewise increases with decreasing temperatures. This is considering book differs according to temperature. Volume increases with increasing temperature. If you are curious as to why the density of a pure substance could vary with temperature, check out the ChemWiki page on Van Der Waal interactions. Beneath is a tabular array showing the density of pure water with differing temperatures.
| Temperature (C) | Density (g/cm3) |
|---|---|
| 100 | 0.9584 |
| lxxx | 0.9718 |
| lx | 0.9832 |
| xl | 0.9922 |
| 30 | 0.9957 |
| 25 | 0.997 |
| 22 | 0.9978 |
| 20 | 0.9982 |
| 15 | 0.9991 |
| x | 0.9997 |
| 4 | 1.000 |
| 0 (liquid) | .9998 |
| 0 (solid) | 0.9150 |
As can be seen from Tabular array \(\PageIndex{ii}\), the density of h2o decreases with increasing temperature. Liquid h2o besides shows an exception to this rule from 0 degrees Celsius to iv degrees Celsius, where it increases in density instead of decreasing every bit expected. Looking at the table, you can too run across that ice is less dense than water. This is unusual every bit solids are more often than not denser than their liquid counterparts. Ice is less dense than water due to hydrogen bonding. In the water molecule, the hydrogen bonds are strong and meaty. As the h2o freezes into the hexagonal crystals of water ice, these hydrogen bonds are forced farther apart and the volume increases. With this volume increase comes a decrease in density. This explains why water ice floats to the top of a loving cup of water: the water ice is less dumbo.
Even though the rule of density and temperature has its exceptions, it is still useful. For example, it explains how hot air balloons work.

Density and Force per unit area
Density increases with increasing pressure because volume decreases as pressure increases. And since density=mass/book , the lower the volume, the higher the density. This is why all density values in the Periodic Table are recorded at STP, as mentioned in the section "Density and the Periodic Table." The subtract in volume equally related to pressure is explained in Boyle's Law: \(P_1V_1 = P_2V_2\) where P = pressure and 5 = volume. This idea is explained in the figure below. More about Boyle's Law, as well as the other gas laws, can exist found here.
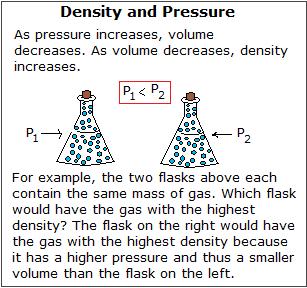
Archimedes' Principle
The Greek scientist Archimedes made a pregnant discovery in 212 B.C. The story goes that Archimedes was asked to find out for the King if his goldsmith was cheating him by replacing his gold for the crown with silver, a cheaper metallic. Archimedes did non know how to find the volume of an irregularly shaped object such equally the crown, even though he knew he could distinguish between elements past their density. While meditating on this puzzle in a bath, Archimedes recognized that when he entered the bath, the water rose. He then realized that he could use a similar process to determine the density of the crown! He and then supposedly ran through the streets naked shouting "Eureka," which means "I found it!" in Latin.
Archimedes so tested the king'south crown by taking a 18-carat gold crown of equal mass and comparison the densities of the two. The king'southward crown displaced more water than the gold crown of the aforementioned mass, meaning that the male monarch'south crown had a greater volume and thus had a smaller density than the real gold crown. The king'southward "golden" crown, therefore, was non fabricated of pure golden. Of course, this tale is disputed today because Archimedes was non precise in all his measurements, which would brand it hard to determine accurately the differences between the two crowns.
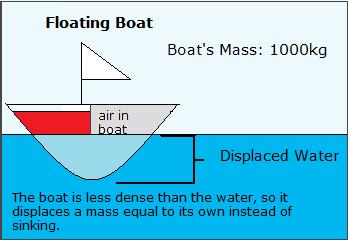
Archimedes' Principle states that if an object has a greater density than the liquid that it is placed into, it will sink and displace a book of liquid equal to its own. If information technology has a smaller density, it volition float and readapt a mass of liquid equal to its own. If the density is equal, it will non sink or float. This principle also explains why balloons filled with helium float. Balloons, as we learned in the section apropos density and temperature, float because they are less dense than the surrounding air. Helium is less dense than the atmospheric air, so it rises. Archimedes' Principle can also be used to explain why boats bladder. Boats, including all the air infinite, inside their hulls, are far less dense than h2o. Boats made of steel tin can float considering they displace their mass in h2o without submerging all the way.
Table \(\PageIndex{3}\) beneath gives the densities of a few liquids to put things into perspective.
| Liquid | Density in kg/thou3 | Density in g/cmiii |
|---|---|---|
| 2-Methoxyethanol | 964.threescore | 0.9646 |
| Acetic Acrid | 1049.x | 1.049 |
| Acetone | 789.86 | 0.7898 |
| Booze, ethyl | 785.06 | 0.7851 |
| Booze, methyl | 786.51 | 0.7865 |
| Ammonia | 823.35 | 0.8234 |
| Benzene | 873.81 | 0.8738 |
| Water, pure | m.00 | one.000 |
Pct Composition
Percent composition is very simple. Percent limerick tells yous by mass what percent of each element is present in a chemical compound. A compound is the combination of 2 or more than elements. If you are studying a chemical compound, you may want to discover the per centum composition of a certain chemical element within that chemical compound. The equation for percent composition is (mass of element/molecular mass) x 100.
Steps to computing the percent composition of the elements in an compound
- Detect the molar mass of all the elements in the chemical compound in grams per mole.
- Discover the molecular mass of the unabridged compound.
- Split up the component's molar mass by the entire molecular mass.
- You will now take a number between 0 and 1. Multiply it by 100% to become percent limerick.
Tips for solving:
- The percentage composition of all elements in a compounds must add upward to 100%. In a binary compound, you can discover the % of the starting time element, then do 100%-(% start chemical element) to get (% second element)
- If using a calculator, you can store the overall molar mass to a variable such as "A". This volition speed up calculations, and reduce errors.
Example \(\PageIndex{1}\): Phosphorus Pentachloride
What is the percent composition of phosphorus and chlorine in \(PCl_5\)?
Solution
Detect the molar mass of all the elements in the compound in grams per mole.
- \(P\): \(1 \times xxx.975 \,g/mol = 30.75\, g/mol\)
- \(Cl\): \(5 \times 35.453 \, g/mol = 177.265\, g/mol\)
Find the molecular mass of the entire compound.
- \(PCl_5\): \(ane \times 30.975 \,g/mol + 5 \times 35.453 \, 1000/mol = 208.239 \, g/mol\)
Split up the component'south molar mass by the entire molecular mass.
- \(P\): \(\dfrac{30.75 \, grand/mol}{208.239\, g/mol} \times 100\% = 14.87\%\)
- \(Cl\): \(\dfrac{177.265 \, g/mol}{208.239\, g/mol} \times 100\% = 85.thirteen \%\)
Therefore, in \(PCl_5\) is 14.87% phosphorus and 85.xiii% chlorine by mass.
Example \(\PageIndex{two}\): HCl
What is the percent composition of each chemical element in hydrochloric acid (HCl).
Solution
First notice the tooth mass of hydrogen.
\[H = 1.00794 \,g\]
At present find the molecular mass of the HCl molecule:
\[1.00794\,g + 35.4527\,chiliad = 36.46064\,thousand\]
Follow steps 3 and iv:
\[ \left(\dfrac{1.00794\,k}{36.46064\,g}\right) \times 100\% = 2.76\% \]
Now just subtract to detect the per centum past mass of chlorine in the chemical compound:
\[100\%-ii.76\% = 97.24\%\]
Therefore, \(HCl\) is 2.76% hydrogen and 97.24% chlorine by mass.
Pct Composition in Everyday Life
Per centum composition plays an important office in everyday life. It is more than just the amount of chlorine in your swimming puddle because it concerns everything from the money in your pocket to your health and how you lot live. The next 2 sections describe percentage limerick as it relates to you lot.
Nutrition Labels
The diet characterization establish on the container of every flake of processed nutrient sold by the local grocery store employs the thought of per centum composition. On all diet labels, a known serving size is broken down in 5 categories: Total Fatty, Cholesterol, Sodium, Total Carbohydrate, and Protein. These categories are broken downwardly into further subcategories, including Saturated Fat and Dietary Cobweb. The mass for each category, except Poly peptide, is then converted to pct of Daily Value. Only 2 subcategories, Saturated Fatty and Dietary Fiber are converted to percent of Daily Value. The Daily Value is based on a the mass of each category recommended per day per person for a 2000 calorie diet. The mass of protein is not converted to percentage because their is no recommended daily value for poly peptide. Following is a moving-picture show outlining these ideas.
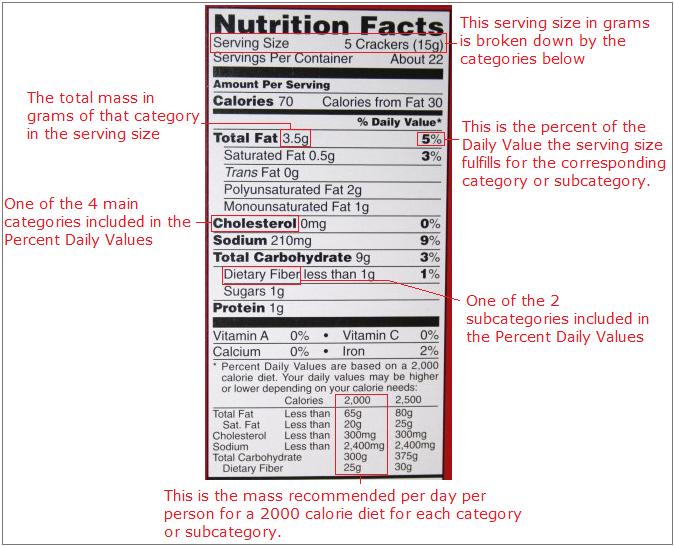
For example, if you wanted to know the percent by mass of the daily value for sodium you are eating when you consume one serving of the food with this nutrition label, then go to the category marked sodium. Look beyond the same row and read the percent written. If you swallow one serving of this food, then yous will have consumed nigh 9% of your daily recommended value for sodium. To find the percentage mass of fat in the whole food, you lot could split 3.v grams by xv grams, and come across that this snack is 23.33% fatty.
Penny: The Lucky Copper Coin
The penny should exist called "the lucky copper coated coin." The penny has not been made of solid copper since part of 1857. After 1857, the United states regime started adding other cheaper metals to the mix. The penny, being but one cent, is literally not worth its weight in copper. People could melt copper pennies and sell the copper for more than than the pennies were worth. Afterwards 1857, nickel was mixed with the more expensive copper. After 1864, the penny was made of bronze. Bronze is 95% copper and 5% zinc and tin. For one yr, 1943, the penny had no copper in information technology due to the expense of the World War II. It was just zinc coated steel. Later 1943 until 1982, the penny went through periods where it was brass or bronze.

Today, the penny in America is ii.5% copper with 97.5% zinc. The copper coats the outside of the penny while the inner portion is zinc. For comparison's sake, the penny in Canada is 94% steel, 1.five% nickel, and 4.5% copper.
The percent composition of a penny may actually bear on health, particularly the wellness of modest children and pets. Since the newer pennies are made mainly of zinc instead of copper, they are a danger to a child's health if ingested. Zinc is very susceptible to acid. If the thin copper coating is scratched and the hydrochloric acid present in the stomach comes into contact with the zinc core it could cause ulcers, anemia, kidney and liver damage, or even decease in severe cases. Three important factors in penny ingestion are time, pH of the stomach, and amount of pennies ingested. Of course, the more than pennies swallowed, the more danger of an overdose of zinc. The more than acidic the surround, the more zinc will be released in less time. This zinc is then captivated and sent to the liver where it begins to crusade damage. In this kind of state of affairs, time is of the essence. The faster the penny is removed, the less zinc is captivated. If the penny or pennies are non removed, organ failure and death can occur.
Below is a movie of a scratched penny before and subsequently it had been submerged in lemon juice. Lemon juice has a similar pH of 1.5-ii.five when compared to the normal human stomach after nutrient has been consumed. Time elapsed: 36 hours.


As you tin see, the copper is vastly unharmed by the lemon juice. That'south why pennies made earlier 1982 with mainly copper (except the 1943 penny) are relatively safety to eat. Chances are they would laissez passer through the digestive organisation naturally before whatever harm could be done. All the same, it is clear that the zinc was partially dissolved even though it was in the lemon juice for only a express amount of time. Therefore, the per centum limerick of post 1982 pennies is hazardous to your health and the health of your pets if ingested.
Summary
Density and percentage composition are of import concepts in chemistry. Each have basic components likewise equally broad applications. Components of density are: mass and book, both of which can exist more confusing than at first glance. An application of the concept of density is determining the volume of an irregular shape using a known mass and density. Determining Percent Limerick requires knowing the mass of entire object or molecule and the mass of its components. In the laboratory, density can be used to identify an element, while percent composition is used to determine the amount, by mass, of each chemical element present in a chemical chemical compound. In daily life, density explains everything from why boats float to why air bubbles will try to escape from soda. It fifty-fifty affects your wellness considering os density is very of import. Similarly, percent composition is unremarkably used to make animal feed and compounds such equally the blistering soda found in your kitchen.
Density Problems
These problems are meant to be easy in the kickoff and so gradually get more challenging. Unless otherwise stated, answers should exist in g/mL or the equivalent g/cm3 .
- If you have a 2.130 mL sample of acerb acid with mass .002234 kg, what is the density?
- Summate the density of a .03020 L sample of ethyl alcohol with a mass of 23.71002 m.
- Find the density of a sample that has a volume of 36.v L and a mass of ten.0 kg.
- Find the book in mL of an object that has a density of 10.2 g/L and a mass of 30.0 kg.
- Summate the mass in grams of an object with a book of 23.5 mL and density of 10.0 k/L.
- Summate the density of a rectangular prism made of metal. The dimensions of the prism are: 5cm by 4cm by 5cm. The metal has a mass of fifty grams.
- Find the denstiy of an unknown liquid in a beaker. The beaker's mass is 165g when there is no liquid present. With the unknown liquid, the full mass is 309g. The volume of the unknown is 125mL.
- Determine the density in g/L of an unknown with the following information. A 55 gallon tub weighs 137.5lb when empty and 500.0 lb when filled with the unknown.
- A ring has a mass of v.00g and a book of 0.476 mL. Is information technology pure silvery?
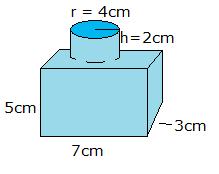
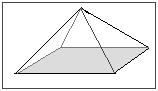
- What is the density of the solid in the image if the mass is xl g? Brand your answer have three pregnant figures.
11) Below is a model of a pyramid found at an archeological dig made of an unknown substance. It is too big to observe the book by submerging it in h2o. Too, the scientists refuse to remove a piece to test because this pyramid is a function of history. Its height is 150.0m. The length of its base is 75.0m and the width is 50.0m. The mass of this pyramid is v.50x105kg. What is the density?
Density Problem Solutions
- 1.049 g/mL
- 0.7851 g/mL
- 0.274 g/mL
- 2.94 10 106 mL
- 0.3.27 kg
- 0.five g/cm3
- 1.15 g/mL
- 790 g/L
- Yep
- 0.195 one thousand/cmthree
- 29.three k/cm3
Percent Composition Issues
These problems will follow the aforementioned blueprint of difficulty as those of density.
- Calculate the percent past mass of each element in Cesium Fluoride (CsF).
- Summate the percent by mass of each chemical element present in carbon tetrachloride (CCl4)
- A solution of salt and water is 33.0% salt past mass and has a density of 1.fifty g/mL. What mass of the salt in grams is in five.00L of this solution?
- A solution of water and HCl contains 25% HCl past mass. The density of the solution is 1.05 g/mL. If you need 1.7g of HCl for a reaction, what volume of this solution will you apply?
- A solution containing 42% NaOH past mass has a density of 1.xxx thou/mL. What mass, in kilograms, of NaOH is in 6.00 Fifty of this solution?
Percent Composition Problem Solutions
- CsF is 87.5% Cs and 12.5% F by mass
- CClfouris 92.2% Cl and 7.eight% C by mass
- 2480g
- vi.5mL
- 2.38 kg
References
- AUTOR , ARQUIMEDES , and Thomas Little . The Works of Archimedes . Courier Dover Publications, 2002.
- Chande, D. and T. Fisher (2003). "Have a Penny? Need a Penny? Eliminating the One-Cent Money from Circulation." Canadian Public Policy/Analyse de Politiques 29(four): 511-517.
-
Jefferson, T. (1999). "A Thought for Your Pennies."
JAMA281(2): 122.
- Petrucci , Ralph , William Harwood , and Geoffrey Herring . Principles and Mod Application. ninth . New Jersey : Peason Eduation , 2007.
-
Rauch, F., H. Plotkin, et al. (2003). "Bone Mass, Size, and Density in Children and Adolescents with Osteogenesis Imperfecta: Effect of Intravenous Pamidronate Therapy."
Journal of Bone and Mineral Researchxviii: 610-614.
-
Richardson, J., South. Gwaltney-Brant, et al. (2002). "Zinc Toxicosis from Penny Ingestion in Dogs."
Vet Med97(2): 96-99.
-
Tate, J. "Archimedes' Discoveries: A Closer Expect."
Source: https://chem.libretexts.org/Bookshelves/Analytical_Chemistry/Supplemental_Modules_(Analytical_Chemistry)/Quantifying_Nature/Density_and_Percent_Compositions
Posted by: marshalluntle1949.blogspot.com


0 Response to "How To Find The Density Of Something"
Post a Comment